
Current position:
Tenure Track, Senior Post-doc
Fields of study:
Computational neurophysics: mean field models, brain dynamics, functional neuroimaging, cortical connectivity
Degrees:
Ph.D. (Dr. rer. nat.) and M.Sc. (Dipl.-Phys.) from the Universität Dortmund (Lehrstuhl Theoretische Physik IV), Germany
Languages:
German (native), English (fluent), Dutch, French
Birth:
25 January 1971 in Wermelskirchen, Germany
Often it is convenient to model the overall behaviour of neural masses rather than of individual neurons. Mean field models do this by coarse-graining over time and/or space, i.e., by ignoring fine temporal and/or spatial detail of neural activity. What is lost in the details is gained in the simplicity of mathematical description and numerical computation. Clearly, whether this simplification is appropriate depends on the sort of problem one is considering. Mean field models work well for the description of noninvasive imaging of brain activity. The best spatial resolution one can expect for functional imaging in the forseeable future is about 1 mm (e.g., from fMRI), roughly the size of a cortical macrocolumn. In such a macrocolumn we find a large number of neurons, typically about 105. It is the overall activity of all these neurons, which will be seen in noninvasive imaging. In the case of the EEG/MEG, the spatial resolution is worse than 1 cm. Realistic simulations of about 107 individual neurons are out of question, so mean field models are the best option.
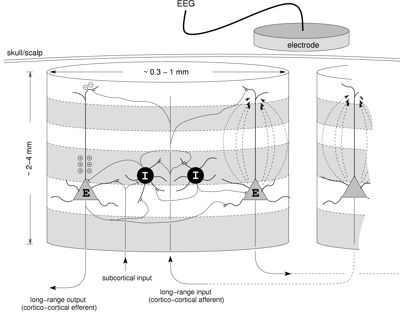
Since the EEG/MEG has excellent temporal resolution but only mediocre spatial resolution, it is appropriate to coarse-grain only in space but not in time. The picture on the right illustrates that the activity detected by the EEG/MEG are synaptically induced currents in the apical dendrites of excitatory neurons, which lead to microscopic current 'dipole' contributions to the potential differences detected on the scalp. When many such contributions add coherently, which is possible since these dendrites are largely aligned in parallel, one can obtain a macroscopic signal. It turns out that the strength of this macroscopic signal is proportional to the modelled mean excitatory membrane potential.
The comparatively simple mean field maths can be used to simulate the brain dynamics of even a full size human cortex [Bojak04/05] on parallel supercomputers. The movie (click to start) shows spontaneously emerging 'alpha rhythm' patterns (final frequency 10.74 Hz with wavelength 17.1 cm). The total 'brain time' simulated is about 18 seconds. Shown by height and color is the strength of the mean excitatory membrane potential, i.e., the EEG signal. The movie is an AVI file with Microsoft MPEG4 V2 as codec and is scaled down to 200 x 150 pixels. The movie is not as high res as the image due to compression, but it remains 5.2 MB large. It should play in most environments.

Our nonlinear mean field model can produce a wide variety of brain dynamics: quasi-linear, limit cycles, chaos, even multi-stability. The movie on the right shows multi-stable attractors (1 chaotic, 2 of limit cycles) which occur in one model parameter set for different starting conditions. It's an AVI file with MPEG4 V2 codec, 400 x 864 pixels, and 2.1 MB large (original data thanks to M. Dafilis). Freeman has speculated that multi-stability could underly cognitive changes.


Due to the richness of dynamics we have to search the parameter space for sets that provide physiologically relevant dynamics. This is complicated by the fact that it is a multidimensional space full of local extrema with regards to the finding of a particular dynamics. One way of searching such a parameter space is with Particle Swarm Optimization [Bojak05]. That algorithm is shown at work on a Griewank test function in the movie above on the left: the absolute minimum is in the center among the many local minima (AVI, MPEG4 V2 codec, 280 x 210 pixels, 2.8 MB). We can use it to find for example reasonable 'alpha sets'.

The picture above shows the power spectra generated by 27 parameter sets we have found with Particle Swarm Optimization [Bojak05]. They have a reasonable 'human-like' shape, i.e., they show a '1/f' fall-off at low frequencies and then an alpha peak with high quality in the range from 8-13 Hz. These sets also yield reasonable firing rates for the 'effective' neurons (between 0.1 and 20/s). While the search used the full nonlinear equations, it turns out that all selected sets could be approximated by a linearization of the equations. Linearization is helpful for much more rapid searches of parameter space, e.g., for generating the close to 80,000 baseline parameter sets for the anaesthesia study described below. As for the resting (alpha) state of the brain,one generally finds that mean field models are well capable of describing key features of brain activity observed with the EEG/MEG in particular.
Mean field models can bridge the mesoscopic gap between our knowledge of the (sub)cellular biochemical and -electrical activity of neurons and the coherent actions of many neurons observable with noninvasive modalities. General anaesthetic agents interact with a range of ligand-gated ionic channels and affect the presynaptic neurotransmitter release and the postsynaptic ionic current flow. These effects can be modelled through changes of the parameters in the model that correspond to the postsynaptic potential occuring after a presynaptic pulse. With the variation of parameters the EEG activity derived from the model can change as well, thus predicting the EEG under anaesthesia through the model.
In [Bojak04] we varied a parameter that corresponds to the duration of the inhibitory response, which is a reasonable approximation to the action of many anaesthetics. As seen in the picture to the right (blue line), we have first kept the simulated cortex at the normal parameter value for 5 seconds, then simulated induction by prolonging the inhibitory response slowly over 10 seconds, next kept 'full anaesthesia' for 5 seconds, then shortened the inhibitory response again over 10 seconds for recovery, and finally kept the cortex at normal parameter value for another 5 seconds. The Fourier spectrum of the model cortex shows a biphasic power response in the 3.9 to 7.8 Hz band.

The movie (click to start) shows a simulation of anaesthesia as described above. All patterns emerge under noise input (see [Bojak05] for details on the noise shaping). The scale with the moving pointer at the bottom right corresponds to time in the plot above, i.e., its left end corresponds to 0 seconds, its right end to 35 seconds. Shown by height and color is the strength of the mean excitatory membrane potential across a full size (51.2 cm x 51.2 cm) cortex. The movie is an AVI file with Microsoft MPEG4 V2 as codec and is scaled down to 200 x 150 pixels. The movie is not as high res as the image due to compression, but it remains 15.5 MB large. It should play in most environments.

Originally postsynaptic potentials (PSPs) were modelled with an 'alpha' form. This is not sufficient for describing actual experimental data, since in the 'alpha' form the rise time of the PSPs is coupled to the decay time. In [Bojak05] we introduced a new type of bi-exponential form for the PSPs, which allowed us to vary rise and decay time separately. Using this new form we were able to fit experimental data for the action of the volatile anaesthetic agent isoflurane on the PSPs. The figure to the right shows anaesthetic action on the inhibitory on inhibitory PSP (left) and excitatory on excitatory PSP (right). Note how the the decay time on the left increases strongly with concentration of the anaesthetic agent without affecting the rise time.The movie to the right (click to start) shows the Fourier power spectrum of a simulation with the improved PSPs.


In the movie above concentration of isolurane is varied from 0 to 3.33 MAC. Maintenance level during surgery is typically 1.3 to 3 MAC. [Number on top: MAC, ordinate: frequency (0 Hz bottom, 30 Hz top), abscissa: inverse wavelength (0/cm left, 0.442/cm right), color: normalized spectral power.] Watch how the alpha band (red band in the middle) drops with higher anaesthesia concentrations. One can also see the biphasic power rise as transient change to more red color. The movie above is an AVI file with Microsoft MPEG4 V2 as codec and is scaled down to 100 x 400 pixels. It is 3.1 MB large and should play in most environments.

The picture above shows close to 80,000 different parameter sets which all display a good resting alpha spectrum, see top half of the picture. One line parallel to the ordinate displays one alpha spectrum, color-coded according to strength of spectral power, whereas along the abscissa the almost 80,000 spectra are sorted in according to their central alpha frequency. The lower half of the picture shows the effect of 2 MAC isoflurane. The universal drop of the alpha band to low frequencies and its broadening is easy to discern. This corresponds well to the changes observed in real EEG under anaesthesia. That all parameter sets show the correct behaviour demonstrates that the model has captured an essential part of the underlying physiological realities.
The physiological mechanisms that underlie the transition to seizure in epilepsy are still largely unknown. In brain dynamics epilepsy is often modelled with limit cycle behaviour. Stable limit cycles are self-sustained oscillations of a system, which are predictable and stable against small disturbances (but not generally sinusoidal). However, limit cycles are ubiquitous in nonlinear systems. Since we lack a thorough understanding of ictogenesis, finding a limit cycle that looks like epilepsy does not by and in itself imply that the nonlinear system under investigation is providing a meaningful physiological description of epilepsy.
It is perhaps counter-intuitive, but some anaesthetic agents can cause seizure activity in the induction phase. As the table to the right shows, enflurane in particular is know to be proconvulsant. Since we can model the EEG under anaesthesia, this provides an opportunity for modelling epilepsy meaningfully. We can track the changes of the model under anaesthesia and find out whether it will display limit cycle activity at some stage. We are then following known physiological changes and hence avoid the interpretation issue that generally plague the association of limit cycles with epilepsy. We will compare enflurane with isoflurane, which does not appear to be proconvulsant.

The movie (click to start) shows a simulation of an induction with enflurane [Liley05]. All patterns emerge spontaneously under noise input [Bojak05]. Shown by height and color is the strength of the mean excitatory membrane potential across a full size (51.2 cm x 51.2 cm) cortex. The total 'brain time' of the simulation is two seconds. The movie is an AVI file (Microsoft MPEG4 V2 codec, scaled down to 200 x 150 pixels, compressed to 2.8 MB with resolution loss). It should play in most environments. We can see how seizure activity arises locally and then spreads across the entire cortex, reminding us of the secondary generalization of partial epileptic activity.

We can now look at different model parameter sets (all with physiological 'alpha' baseline), which represent different patients with somewhat different physiology and current brain state. We vary two parameters, the amplitude of the inhibitory postsynaptic potential and its decay time, according to data on the action of enflurane (solid curve) and isoflurane (dashed curve), respectively. The markers on the curvers show increases agent concentration of 0.5 vol%. Two different parameter sets, (a) and (b), are shown for illustration. The white region in the plots indicates regular (alpha) activity, the grey region indicates epileptic activity (large amplitude limit cycle with low frequencies). We see that the enflurance curve crosses into the ictal region for parameter set (a), but not for (b). The isoflurane curve always stays in the regular region. Technically, crossing into the seizure region generally occurs via a supercritical Hopf bifurcation.

In investigating a large number of parameter sets we found that with enflurane a significant fraction would similarly undergo seizure, but with isoflurane none. This corresponds well with the clinical and research data concerning the different proconvulsant properties of these agents, as summarized in the table above. We also found that destablization typically occured at 2-3 vol% of enflurane, which also compares well with observations. Thus we can say that we understand the underlying physiological basis of one particular path to epilepsy in terms of the destabilization of our mean field model through changes to inhibitory postsynaptic potentials. Whether this study opens a window also on other, spontaneous, modes of epilepsy remains to be seen.
Experimentally it is found that a wide variety of cognitive activity in animals and humans leads to an increase of electrocortical brain activity in the gamma range (typically around 40 Hz). This increased activity appears within 100 to 300 ms of task onset. Not only do physically separate areas of the brain enhance their gamma output, it is found that these different areas actually show synchrony of their activity, i.e., the oscillations are in phase. This has lead to the suggestion that gamma synchrony is a sign of distant neural assemblies being 'bound together' in order to cooperate on a cognitive task. Can mean field models provide some further insights into these experimental results [Bojak07]?
The picture on the right shows a so-called 'bifurcation diagram'. It provides a graphical description of the system dynamics. The 'bifurcation parameter' is shown in percent of regular value on the abscissa. At 100% the system oscillates as driven by input around a 'fixed point' (blue line) of the mean excitatory membrance potential shown on the ordinate. If the system is driven across the 'unstable periodic' red line, self-sustained oscillations set in between the 'stable periodic' green lines. However, for a 4.7% or more increase of inhib.-inhib. connections, the state becomes 'unstable' (purple line), and develops self-sustained oscillations independent of the driving input [Bojak07].

The movie (click to start) shows a full size (51.2 cm x 51.2 cm) cortex, which has been slightly destabilized by increasing the number of inhib.-inhib. connections. Color shows the strength of the mean excitatory membrane potential (EEG). 'Brain time' of the simulation is 1.5 seconds. The movie is an AVI file with Microsoft MPEG4 V2 codec, 400 x 400 pixels, compressed to 5.5 MB with resolution loss. It should play in most environments. We can see how 'hot spots' of activity emerge spontaneously across the cortex. The first and the second 'hot spot' have a distance of about 6.4 cm and emerge within 100 ms of each other. The third 'hot spot' has a distance of about 29.5 cm to the first two (toroidal connectivity), and emerges 30 ms later. The input is merely noise, none of the structures is imprinted. The whole cortex fills up here because all of it (rather than specific regions) has been destabilized here.

What kind of activity do we see in the hotspots? We take a 3D-Fourier transform of the data show above and collapse the two spatial Fourier dimensions into one by seeking the maxium on a radial sweep. The movie shows the resulting 2D power spectrum according to frequency and (radial) wavelength. It is an AVI file (Microsoft MPEG4 V2 codec, 400 x 300 pixels, 0.7 MB). We can see that the resting state alpha activity increases in amplitude until the 'hot spots' begin to emerge. Then we see transient low frequency behaviour followed by a rapid switch to a strong dominance of activity at 36 Hz with finite (radial) wavelength. Thus these are 'gamma hot spots'.

How about synchrony? We perform an analysis using Hilbert phase locking. To this end we compute the Hilbert instantaneous phase for every point on cortex. The Hilbert phase generalizes phase comparisons from simple sine waves to more complex time series. Next we pick a point in the first emerging gamma hot spot as our reference. Every phase computed elsewhere is then compared to this chosen reference. In practice we compute the distribution of differences in phase over a 0.5 s window. This distribution is the scored from 0 (anti-synchrony, blue) to 1 (perfect synchrony, red) using an entropic synchronization index. Next the window is advanced by 30 ms and the analysis is repeated. The result is shown by the movie (Microsoft MPEG4 V2 codec, 400 x 400 pixels, 2.1 MB). We see that if the entire cortex is destabilized, it will completely synchronize in gamma activity [Bojak07].

The remaining synchrony pattern above corresponds simply to the spatiotemporal motion of the activity, i.e., a synchronized wave will be seen to oscillate between anti- and perfect synchrony with distance as compared to a fixed point. Besides the increase of inhib.-inhib. connectivity also a 12.5% decrease of the amplitude of inhibitory postsynaptic potentials will trigger gamma activity [Bojak07]. It is significant that both ways of destabilizing cortex that have been explored imply relatively minor shifts of parameters, which is biologically plausible. Possible physiological mechanisms for these parameter changes would be changes of the GABA release due to presynaptic connections of thalamic afferents [Bojak07].
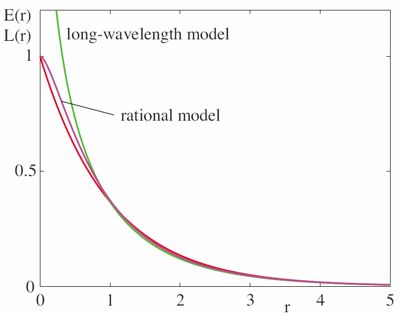
Better modelling of cortico-cortical connectivity is of prime importance for improving the realism of mean field models. One aspect of this is the 'background' (non-specific) connectivity of the brain. So far most mean field models have used an isotropic, homogeneous exponential decay of the connectivity over distance as the underlying assumption, which is the approximated with a 'long-wavelength expansion'. In [Coombes07] we have provided a better approximation.
The fully general form for the connectivity is an integro-differential equation, which is however difficult to solve even numerically. The assumption of exponential 'background connectivity' brings one closer to numerical computability. However, in addition one has to expand the result in small wavenumbers (i.e., long wavelengths). Only then does one obtains a nice partial differential equation (PDE). In [Coombes07] we have provided a different approximation, which we call the 'rational model'. It also yields a computable PDE, but stays much closer to the original exponential assumption in particular at small distances, see the figure to the right (red line is the original).
It is by now known that the neocortex has a crystalline microstructure at the millimeter length scale. So we must revise the assumption of isotropy. The cortical feature maps are sometimes approximately periodic (e.g., in the visual cortex) and this can be modelled with 'patchy connections' that break rotation symmetry but not translation symmetry. Thus we multiply the usual connectivity with some rotationally periodic function. In general this leads to an infinite number of PDEs, but for simple periodicity only a finite number of PDEs obtain. The figure to the right (advancing by quarter period left to right, top to bottom) shows a simulation of activity with the 'rational model' using rotational symmetry broken by patchy connections. We see that lattice-directed waves emerge from a state that was spatially uniform.

Much more works is required to bring realistic connectivity to mean field models, [Coombes07] was just a small first step in that direction. In my opinion, the lack of specific connectivity is the major weakness of the mean field approach for the time being. It is hence one of the main aims of my work in the NeuroPI group to embed experimental connectivity data, e.g., from the inhouse CoCoMac database, into the successful mean field model described above.
April 2007
- present
Senior Postdoctoral Fellow, Tenure Track (5 years), Lecturer, with Prof. R. Kötter at the NeuroPI Group, Department of Cognitive Neuroscience, University Medical Centre St. Radboud, Nijmegen, The Netherlands.
October 2004
- present
Research Consultant, Cortical Dynamics Pty. Ltd., a division of BioPharmica Ltd., Perth, Australia.
October 2002
- March 2007
Postdoctoral Fellow, Lecturer, Associate Supervisor (two Ph.D. Students), with Prof. D.T.J. Liley at the Brain Dynamics Research Unit (formerly CISCP), Brain Science Institute, Swinburne University of Technology, Hawthorn (Melbourne), Australia.
October 2000
- September 2002
Postdoctoral Fellow, with Prof. A.W. Thomas at the ARC Special Research Centre for the Subatomic Structure of Matter, University of Adelaide, Australia.
May 2000
- September 2000
Scientific Assistant, with Prof. E. Reya at the Lehrstuhl Theoretische Physik IV, Universität Dortmund, Germany.
5 May 2000
Ph.D. in theoretical particle physics: summa cum laude, Supervisor: Prof. E. Reya, Lehrstuhl Theoretische Physik IV, Universität Dortmund, Germany.
January 1996
- April 2000
Ph.D. Student in theoretical particle physics with Prof. E. Reya at the Lehrstuhl Theoretische Physik IV, Universität Dortmund, Germany.
6 December 2000
M.Sc. in theoretical particle physics: very good (sehr gut), Supervisor: Prof. E. Reya, Lehrstuhl Theoretische Physik IV, Universität Dortmund, Germany.
October 1992
- December 1995
Masters Student in theoretical particle physics with Prof. E. Reya at the Lehrstuhl Theoretische Physik IV, Universität Dortmund, Germany.
October 1990
- September 1992
Student in (theoretical) physics, Fakultät Physik, Universität Dortmund, Germany.
Reviewer
Physical Review E - Statistical, Nonlinear, and Soft Matter Physics, American Physical Society
Reviewer
Cosmos and History - The Journal of Natural and Social Philosophy
Board Member
Directors of CNS - Organization for Computational Neurosciences
Member
DPG - Deutsche Physikalische Gesellschaft
Member
EPS - European Physical Society
previous Member
Biomedical Program Group - coordinates biomedical studies at Swinburne University of Technology, Hawthorn, Australia
previous Staff Representative
State Doctorate (Habilitation) committee - Fakultät Physik, Universität Dortmund, Germany
2007
Lecturer in 3rd year bachelor course for biology / molecular life sciences: 'Neuroscience: van basis tot kliniek', Radboud University, Nijmegen, The Netherlands.
2007
Tutor in biophysics labs: 'Audiometrie', 'Ioniserende straling', '(Ultra)geluid / Stroming / Elesticiteit', 'Gastransport / Beeldvorming', at Radboud University, Nijmegen, The Netherlands.
2005 - 2007
Associate Supervisor for two neuroscience Ph.D. students (main Supervisor: Prof. D.T.J. Liley) at Swinburne University of Technology - one Ph.D. project was of mathematical nature (bifurcation analysis of nonlinear brain dynamics), the other experimental (effects of nitrous oxide on the EEG).
2005
Convenor of HET408 Biomedical Imaging and Emerging Technologies for final year students at Swinburne University of Technology, Hawthorn (Melbourne), Australia.
2005 - 2006
Lecturer HET419 Physiological Modelling for final year students at Swinburne University of Technology, Hawthorn (Melbourne), Australia.
2003 - 2007
Lecturer HET408 Biomedical Imaging and Emerging Technologies for final year students at Swinburne University of Technology, Hawthorn (Melbourne), Australia.
1994 - 2000
Teaching Assistant in Advanced Quantum Mechanics and Elementary Particle Theory for physics majors, General and Quantum Physics for other majors at the Fakultät Physik, Universität Dortmund, Germany.
1991 - 1993
Founder of student-led first year physics tutorials, Tutor in Classical Mechanics, Electrodynamics, Special Relativity, and Advanced Maths - later recognized officially by the Fakultät Physik, Universität Dortmund, Germany.
July 2006
Research visit to Prof. K.J. Friston, Wellcome Trust Centre for Neuroimaging, University College London, UK.
2004 - 2007
Teaching grant for biomedical lectures from BioPharmica Ltd., Perth, Australia
August 2000
Research visit to Dr. M. Stratmann, then at the Centre of Particle Theory, University of Durham, UK.
1997 - 2000
Membership in the Graduate Group 'Production and Decay of Elementary Particles' of the German Research Foundation (Deutsche Forschungsgemeinschaft) at the Universität Dortmund, Germany
1992 - 1995
Scholarship from the German National Merit Foundation (Studienstiftung des Deutschen Volkes)
1991 - 1993
Recognition by the Fakultät Physik, Universität Dortmund, Germany and funding by the Northrhine-Westfalia state government of my tutorials as exemplary student initiative
[Coombes07] S. Coombes, N.A. Venkov, L. Shiau, I. Bojak, D.T.J. Liley, and C.R. Laing, Modeling electrocortical activity through improved local approximations of integral neural field equations, Physical Review E 76 (2007) 051901, 1-8, also Virtual Journal of Biological Physics Research 14 (10), 2007. (alternative link)
[Bojak07] I. Bojak and D.T.J. Liley, Self-organized 40 Hz synchronization in a physiological theory of EEG, Neurocomputing 70 (2007) 2085-2090. (alternative link)
[Liley05] D.T.J. Liley and I. Bojak, Understanding the transition to seizure by modeling the epileptiform activity of general anesthic agents (invited article), Journal of Clinical Neurophysiology 22 (2005) 300-313. (alternative link)
[Bojak05] I. Bojak and D.T.J. Liley, Modeling the effects of anesthesia on the electroencephalogram, Physical Review E 71 (2005) 041902, 1-22, also: Virtual Journal of Biological Physics Research 9 (8), 2005. (alternative link)
[Bojak04] I. Bojak, D.T.J. Liley, P.J. Cadusch and K. Cheng, Electrorhythmogenesis and anaesthesia in a physiological mean field theory, Neurocomputing 58-60 (2004) 1197-1202 (alternative link)
[Bojak03a] I. Bojak and M. Stratmann, Next-to-Leading Order QCD Corrections to the Polarized Hadroproduction of Heavy Flavors, Physical Review D 67 (2003) 034010, 1-9 (alternative link).
[Bojak03b] I. Bojak, Polarized Hadro- and Photoproduction of Heavy Quarks in NLO QCD, International Journal of Modern Physics A 18 (2003) 1477-1480.
[Bojak99a] I. Bojak, Towards polarized hadroproduction of heavy quarks, Nuclear Physics B (Proc. Supp.) 79 (1999) 599-601.
[Bojak99b] I. Bojak and M. Stratmann, Photoproduction of Heavy Quarks in Next-to-Leading Order QCD with Longitudinally Polarized Initial States, Nuclear Physics B 540 (1999) 345-381. (alternative link)
[Bojak98] I. Bojak and M. Stratmann, Next-to-Leading Order QCD Corrections to the Polarized Photoproduction of Heavy Flavors, Physics Letters B 433 (1998) 411-418. (alternative link)
[Bojak97a] I. Bojak and M. Ernst, Limitations of small x resummation methods from F2 data, Nuclear Physics B 508 (1997) 731-752. (alternative link)
[Bojak97b] I. Bojak and M. Ernst, Small x resummations confronted with F2(x,Q2) data, Physics Letters B 397 (1997) 296-304. (alternative link)
[Bojak96] I. Bojak and M. Ernst, Balitskii-Fadin-Kuraev-Lipatov equation versus data from DESY HERA, Physical Review D 53 (1996) 80-88. (alternative link)
I. Bojak, Rhythms of brain activity: Anaesthesia, synchrony, nonlinearity, multimodality, invited by Prof. D. Vaitl, Bender Institute of Neuroimaging , Justus Liebig University Giessen, Germany, January 2008.
I. Bojak, Computational Brain Models of EEG / MEG and fMRI signals in health and disease, invited by Prof. J. van Pelt, Inauguration of the INCF National Node Neuroinformatics of the Netherlands, ZonMw Building, Den Haag, The Netherlands, December 2007.
I. Bojak, Towards a theory-driven analysis of EEG/MEG + fMRI, invited by Prof. D.G. Norris, F.C. Donders Centre for Cognitive Neuroimaging, Radboud University Nijmegen, The Netherlands, November 2007.
I. Bojak, Simulating brain activity: EEG rhythms and beyond, invited by Dr. R. Jurgelenaite, Computing Science Institute Nijmegen Information and Knowledge Systems (IRIS), Radboud University Nijmegen, The Netherlands, October 2007.
I. Bojak, Electrocortical rhythms: Anesthesia, epilepsy, synchrony, invited by Dr. P. Fries, F.C. Donders Centre for Cognitive Neuroimaging, Radboud University Nijmegen, The Netherlands, August 2007.
I. Bojak, Electrocortical rhythms: Anesthesia, epilepsy, synchrony, invited by Dr. J. Goossens, Department of Medical Physics and Biophysics, Radboud University Nijmegen, The Netherlands, May 2007.
I. Bojak, Cortical rhythms: Anesthesia, epilepsy, synchrony, invited by Prof. V. Jirsa, Laboratoire Mouvement et Perception, CNRS et Université de la Mediterranée, Marseille, France, July 2006.
I. Bojak, Cortical rhythms: Anesthesia, epilepsy, synchrony, invited by Prof. N. Cohen, Biosystems Research Group, University of Leeds, UK, July 2006.
I. Bojak, Cortical rhythms: Anesthesia, epilepsy, synchrony, invited by Prof. S. Coombes, Centre for Mathematical Medicine and Biology, University of Nottingham, UK, July 2006.
I. Bojak, Cortical rhythms: Anesthesia, epilepsy, synchrony, invited by Prof. C. van Leeuwen, Perceptual Dynamics Lab, and Prof. Yamaguchi, Laboratory for Dynamics of Emergent Intelligence, RIKEN Brain Science Institute, Wako City, Japan, July 2006.
I. Bojak, Overview: Electrocortical rhythms and anesthesia, invited by Prof. T. Hendtlass, Research Program for Complex Intelligent Systems, Swinburne University of Technology, Hawthorn, Australia, June 2005.
I. Bojak, Electrocortical rhythms and anesthesia, invited by Prof. A.G. Williams, Special Research Centre for the Subatomic Structure of Matter , University of Adelaide, Australia, May 2005.
I. Bojak, D.T.J. Liley, and P.J. Cadusch, Electrorhythmogenesis and anaesthesia in a physiological mean field theory, invited by Prof. K.J. Friston, Wellcome Trust Centre for Neuroimaging, University College London, UK, July 2003.
I. Bojak, D.T.J. Liley, and P.J. Cadusch, A space-averaged cortical mean field theory of the human EEG, invited by Prof. S. Makeig, Swartz Center for Computational Neuroscience, University of California San Diego, USA, June 2003.
Also earlier talks on high energy physics at Universität Wuppertal, Germany; Vrije Universiteit Amsterdam, The Netherlands; Lunds Universitet, Sweden; Brookhaven National Laboratory, USA; and Michigan State University, USA.
I. Bojak, Cortical rhythm of cognition: Self-organized 40 Hz synchronization, invited talk at the CSSM Workshop on QCD and the Strong Interactions, Adelaide, 25-29 September 2006.
I. Bojak and D.T.J. Liley, General anaesthetic agents: From (sub)cellular action to quantitative EEG, abstract for the 14th Australasian Society for Psychophysiology Conference, Melbourne, Australia, 10-12 December 2004, Australian Journal of Psychology 57 (2006) Suppl. 1, 16-40.
I. Bojak, D.T.J. Liley, and P.J. Cadusch, Electrorhythmogenesis and anaesthesia in a physiological mean field theory, talk at the Workshop on nonlinear spatio-temporal neural dynamics - Experiments and Theoretical Models, Alicante, Spain, 8 July 2003.
I. Bojak, proceedings of the 15th International Symposium on High Energy Spin Physics, Long Island, New York, 9-14 September 2002, AIP Conference Proceedings 675 (2003) 338-342.
I. Bojak, proceedings of the Workshop on Physics at the Japan Hadron Facility, Adelaide, Australia, 14-21 March 2002, pp. 212-221, World Scientific, 2002.
I. Bojak, proceedings of the RIKEN BNL Research Center Workshop on Spin Physics at RHIC in Year-1 and Beyond, Brookhaven, USA, 14-18 May 2001, BNL-52635, Volume 33, pp. 45-50.
I. Bojak, proceedings of the 9th International Workshop on Deep Inelastic Scattering, Bologna, Italy, 27 April - 1 May 2001, pp. 543-548, World Scientific, 2002.
I. Bojak, proceedings of the Workshop on Lepton Scattering, Hadrons and QCD, Adelaide, Australia, 26 March - 6 April 2001, pp. 96-101, World Scientific, 2001.
I. Bojak, talk at the Visit of International Advisory Committee to CSSM, Adelaide, Australia, 15-16 November 2001.
I. Bojak, talk at the Workshop on Polarized & Unpolarized Deep Inelastic Lepton-Hadron Scattering, Leiden, Netherlands, 17-28 July 2000.
I. Bojak, proceedings of the RIKEN BNL Research Center Workshop on Predictions and Uncertainties for RHIC Spin Physics, Brookhaven, USA, 6-31 March 2000, BNL-52596, Volume 27, pp. 129-134.
I. Bojak, proceedings of the 13th International Symposium on High Energy Spin Physics, Protvino, Russia, 8-12 September 1998, pp. 309-311, World Scientific, 1999.
2nd IRIS and MiniFab seminar on Advances in Medical Imaging & Diagnostics, Melbourne, Australia, 23 March 2004.
1st IRIS and MiniFab seminar on Advances in Medical Imaging & Diagnostics, Melbourne, Australia, 13 November 2003.
12th Australasian Society for Psychophysiology Conference and 6th Australian Functional Brain Mapping Symposium, Sydney, Australia, 29 November - 3 December 2002.
WISER Workshop on High Performance Computing in Space Environment Research, Adelaide, Australia, 29 July-2 August 2002.
World Space Environment Forum, Adelaide, Australia, 22-25 July 2002.
6th International Workshop on Deep Inelastic Scattering and QCD, Brussels, Belgium, 4-8 April 1998.
DESY Theory Workshop 1997: Recent Developments in QCD, DESY, Hamburg, 24-26 September 1997.
I. Bojak, NLO QCD corrections to the polarized photo- and hadroproduction of heavy quarks, Ph.D. (Dr. rer. nat.) thesis, Readers: Prof. E. Reya and Dr. habil. W. Vogelsang, University of Dortmund, Germany, 2000. [Awarded: excellent (ausgezeichnet: best possible mark for Ph.D.)]
I. Bojak, Gluonen und Strukturfunktionen bei kleinen Bjørken x (Gluons and structure functions at small Bjørken x), M.Sc. (Dipl.-Phys.) thesis, Readers: Prof. E. Reya and Prof. M. Glück, University of Dortmund, Germany, 1995. [Awarded: very good (sehr gut: best possible mark for M.Sc.)]

Prof. Rolf Kötter
Neurophysiology and Neuroinformatics Group
Dept. of Cognitive Neuroscience (126)
University Medical Centre St. Radboud
Nijmegen
The Netherlands

Dr. Dirk Schubert
Neurophysiology and Neuroinformatics Group
Dept. of Cognitive Neuroscience (126)
University Medical Centre St. Radboud
Nijmegen
The Netherlands

Prof. David T.J. Liley
Brain Science Institute
Swinburne University of Technology
Hawthorn (Melbourne)
Australia

Prof. Lennaert van Veen
Dept. of Mathematics and Statistics Concordia University
Montreal
Canada

Prof. Stephen Coombes
Centre for Mathematical Medicine and Biology
University of Nottingham
Nottingham
United Kingdom

Prof. Thom Oostendorp
Dept. of Medical Physics and Biophysics Radboud University
Nijmegen
The Netherlands

My beautiful wife Maria Estela Duque, my cute son Pavel, and yours truly.
Mail address
Department of Cognitive Neuroscience (126)
Radboud University Nijmegen Medical Centre
Postbus 9101
6500 HB Nijmegen
The Netherlands
Location
Room 0.34 (sign: 'Neurofysiologie / Neuroinformatica')
Route 126
Preklinisch Instituut (Gebouw M205), 'Lange Gang'
Geert Grooteplein 21
6525 EZ Nijmegen
The Netherlands
Phone
International: ++31-24/36-68577
The Netherlands: 024/36-68577
Fax
International: ++31-24/35-41435
The Netherlands: 024/35-41435
(The above email address is an image, copy it by hand.
I apologize for the inconvenience caused by this anti-spam measure.)