
Current position :
Birthplace:
Alma mater:
Field of study:
Postdoctoral Researcher
Tiel, The Netherlands
Wageningen UR (MSc), Utrecht University (PhD)
Behavioural Neuroscience, Developmental Biology
Although neurodevelopmental disorders have been described for quite some time, most of the treatments are still focusing on symptom removal, while the underlying molecular mechanisms for the development of these distinct pathways are still elusive. The cerebral cortex in general and the prefrontal cortex (PFC) in particular is involved in the pathology of many of these neurodevelopmental disorders (e.g. Readler et al., 1998). The intricate organization of specialized areas in the mature cortex is achieved by coordinate programs in cells that seed this structure during development. Based upon intrinsic expression of (transcription) factors, morphology, physiological properties and connectivity of cells within a cortical layer, distinction can be made between the different cortical areas. However, the precise temporal aspects for this complex developmental script remain unclear.
Therefore, my current research is aimed at understanding the development of two cortical areas which will eventually help us to better comprehend the etiology of neurodevelopmental disorders.
Unraveling the mechanisms of development of two cortical areas.
Cells of the developing cerebral cortex are generated in a precise temporal pattern that is translated into the spatial layout of the embryonic cerebral wall, such that immature, dividing cells populate the deeper layers and more mature, post-mitotic cells reside in the more superficial layers (Kolk et al., 2006). In addition to this horizontal layering, the cerebral cortex can be divided in anatomically distinct areas reflecting different functions (Miller et al., 2006). Layer V pyramidal cells are the main cortical output neurons and we know now that they exist in different morphological and functional classes in two sublayers of the rat somatosensory cortex (S1) (Schubert et al., 2001; 2006). Yet, whether the heterogeneity between classes can be explained by birth-date is not known.
To follow the development of layer V pyramidal cells at an exact developmental time-point within a certain brain area in vivo, cells will be transfected at exactly E15.5 with a fluorescent marker (GFP). To this end, we will take advantage of the novel innovative technique of in utero electroporation (IUE)-mediated gene transfer to monitor proper brain development (Fig. 1; Kolk et al., 2009). We will assess the intrinsic structural and functional cellular characteristics of transfected cells (Kolk et al., 2006; Schubert et al., 2007). For this we will make use of coronal slices of the rat brain in which we perform single or paired whole cell patch clamp recordings of layer V pyramidal neurons in two cortical areas that play a role in neurodevelopmental disorders: the somatosensory (S1) and prefrontal cortex (PFC).
The labeled cells will be physiologically characterized using state-of-the-art in vitro assays. In this way we will be able to compare a structurally and physiologically defined group of cortical cells of the same age within and between different cortical areas. We hypothesize that the characteristics of layer V cells born at an exact developmental time-point are cell-type specific and may differ between the somatosensory and prefrontal cortex.
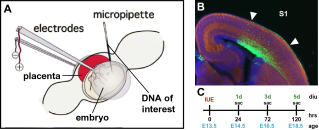
Figure 1 A) Diagram of in utero electroporation (IUE)-mediated gene transfer in mouse embryos B) Low power view of a coronal section through a mouse forebrain electroporated with a control construct (green) in the somatosensory cortex (S1, in between arrowheads) costained with the neuronal marker Tuj1 (red) and fluorescent Nissl (blue). C) Schematic representation of the IUE paradigm to label mouse layer V pyramidal cells. diu, days in utero; hrs, hours; sac, sacrifice).
Elucidating mechanisms of pathological development of the prefrontal cortex.
Cognitive and behavioural impairments seen in the apomorphine-susceptible (APO-SUS) Wistar rats housed at the Central Animal Facility of the Radboud University Nijmegen resemble features of neurodevelopmental disorders. At least part of this phenotype can be attributed to differences in dopaminergic wiring in general, more specifically the mesoprefrontal projections. The IUE technique will be used to again label layer V pyramidal cells within the cortex of APO-SUS versus APO-UNSUS animals at exactly E15.5. Cellular characteristics of both the S1 as well as the PFC will be assessed and compared between SUS and UNSUS animals. Specifically the prefrontal cortex is of interest as it is known that the dopaminergic innervation differs between these two lines affecting the physiological state of layer V pyramidal neurons. We hypothesize that layer V pyramidal cells within the prefrontal cortex born at an exact developmental time-point differ between APO-SUS and APO-UNSUS rats.