Functional and structural aspect of neuronal network formation in health and disease.
The scaffold of proper structural and functional organization of the neuronal networks, such as those in the cortex is depending on the activity of a multitude of different transcription factors, growth factors and neuromodulators during brain development.
Many neurological disorders and their related cortical dysfunctions can be linked with abnormal activity of one or more factors or neuromodulators - be it because of genetic variations or because of pharmacological modulation during critical periods of brain development. The links between brain development and cortical performance in terms of accurate or distorted receiving, processing and sending of information, can be studied very well in the distinctively organized sensory systems and the respective primary sensory cortical areas.
In our group, we focus on the structural and functional organization of the neural networks on two levels and with two translational links:
1: The role of the neuromodulator serotonin on the development of the somatosensory system.
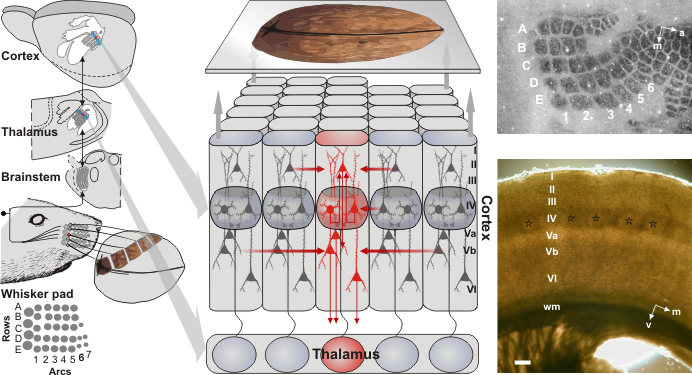
Fig1:Organisation of the rat somatosensory system, taken from Schubert et al., 2007
Serotonin (5-HT) reuptake inhibitors (SSRIs such as Fluoxetine) are commonly prescribed antidepressants that block the serotonin transporter (5-HTT) in neurons and by this can efficiently increase the extracellular levels of 5-HT in the brain. If prescribed to pregnant women, also the unborn foetus is exposed to the SSRis, which is consequently increasing the 5-HT levels during critical phases of brain development.
We use the rat somatosensory cortex of genetically altered rats, in which the 5-HT transporter (in rodents = SERT) is knocked out (SERT-/-), as a model of the consequences of excessive serotonin levels for the development of sensory systems. To this end investigate the alterations from molecular to behavioural level and by this aim to reveal not only the mechanisms underlying 5-HT induced changes in the development of the somatosensory system but also their behavioural consequences.
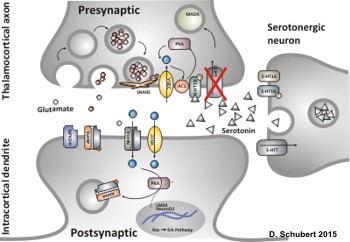
Fig.2: During early postnatal functional dysruption of the transiently expressed SERT in thalamic neurons results in reduced glutamate release at the thalamocortical synapse
2: Epigenetic factors and neuronal network formation in models systems (rodent & human) for intellectual disability.
Kleefstra syndrome is a syndrome caused by the haplosufficiency of EHMT1. It has rapidly emerged as the most prominent subtelomere deletion syndrome giving rise to intellectual disability and autism spectrum disorder (AS). Despite a documented role of EHMT1 in learning and memory behaviour the physopathiology of Kleefstra syndrome remains unknown. EHMT1 is highly expressed in the neocortex during early life. However it is not known how loss of EHMT1 affects the neuronal networks in the neocortex, a brain region that is heavily sculpted through experience-dependent development and whose disruption could explain most deficits found in autism.
Combined with the early postnatal emergence of autism spectrum disorder-related deficits in Kleefstra syndrome and the clinical observations that autistic patients present behaviors consistent with altered sensory processing, we hypothesize that EHMT1 regulates experience-dependent refinement of neuronal circuits during critical phases of brain development. An emerging theme in autism spectrum disorders is that changes in synaptic strength, associated with hyper-connectivity or hypoconnectivity in specific microcircuits e.g. of the somatosensory cortex contributes to the physiopathology of the disease.
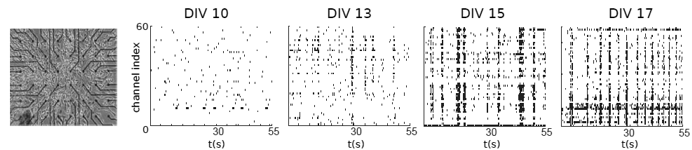
Fig3: Multielectrode recordings from neuronal cultures over time reveal synchronisation of spontaneous networks activity in maturing networks.
Together with the the group of Molecular Neurophysiology (Nadif-Kasri group) we aim to reveal how the lack of EHMT1 affects neuronal network formation and maturation, both on the level of investigating the somatosensory system of EHMT1+/- mice as well as by using neuronal cultures. For the latter we not only have access to the relevant genetically altered mouse lines but also since recently stated to make use of neurons derived from human IPS cells, including patient cell lines.
To this end me make use of rodent acute brain slice preparations as well as neuronal cultures (rodent and, since recently, human IS cell derived).